SEBA Class 9 Science Chapter 2 – Is Matter Around Us Pure Solutions & Summary
Looking for SEBA Class 9 Science Chapter 2 – “Is Matter Around Us Pure” solutions? At Ospin Academy, you will find NCERT-based textbook answers, multiple-choice questions (MCQs), and a detailed chapter summary to help you excel in exams.
📖 Chapter Overview:
This chapter explains the concept of pure substances and mixtures, methods of separation, and different types of solutions.
📌 Key Topics Covered:
- ⭐ Pure Substances and Mixtures
- ⭐ Elements and Compounds
- ⭐ Types of Mixtures: Homogeneous and Heterogeneous
- ⭐ Separation Techniques: Filtration, Evaporation, Distillation, Chromatography
- ⭐ Physical and Chemical Changes
📌 Important Concepts:
- ⭐ What is the difference between pure substances and mixtures?
- ⭐ What are homogeneous and heterogeneous mixtures?
- ⭐ How do we separate components of a mixture?
- ⭐ What is the role of solubility in purification?
- ⭐ What are physical and chemical changes?
📝 How Ospin Academy Helps:
- ✅ Exam-Oriented Solutions: Fully NCERT-based Class 9 Science solutions.
- ✅ MCQs and Extra Questions: Important multiple-choice questions for better revision.
- ✅ Concept Clarity: Explanation with real-life examples.
- ✅ Quick Revision Notes: Key points summarized for last-minute preparation.
Access complete SEBA Class 9 Science Chapter 2 – “Is Matter Around Us Pure” solutions at Ospin Academy and improve your understanding today!
Class 9 Science (English Medium) PDF Solutions 2025-26 | SEBA Assam
Download Class 9 Science (English Medium) PDF with detailed solutions, MCQs, and additional practice questions for SEBA Assam 2025-26.
Class 9 Science
Chapter – 2 (Ospin Academy)
Is Matter Around Us Pure
Page No. 15
1. What is meant by a substance?
Answer:-. A substance is a pure single form of matter. It cannot be separated into other kinds of matter by any physical process. A few examples of substance are sugar, sodium chloride, gold, diamond, etc.
2. List the points of differences between homogeneous and heterogeneous mixtures.
Answer:-. The differences between homogeneous and heterogeneous mixtures are mentioned below:
|
SI NO. |
Basis of difference |
Homogeneous mixture |
Heterogeneous mixture |
|---|---|---|---|
|
(i) |
Composition |
A homogeneous mixture has a uniform composition throughout its mass. |
A heterogeneous mixture does not have a uniform composition throughout its mass. |
|
(ii) |
physical boundaries of separation |
It does not have physically distinct boundaries of separation between the various constituents, e.g. sugar in water. |
It has physically distinct boundaries of separation between the various constituents. The various constituents are visible with naked eyes or at least under microscope, e.g. the mixture of water and oil. |
Page No. 18
1. Differentiate between homogeneous and heterogeneous mixtures with examples.
Answer:-. Refer to Answer of Questions No. 2
2. How are sol, solution and suspension different from each other?
Answer:-. The differences among sol, solution and suspension are are mentioned below:
|
sol |
Solution |
Suspensions |
|---|---|---|
|
A sol is a form of colloidal suspension which has particles with dimensions around 1 nanometer to 1 micrometre. |
A solution is a mixture of two or more substances that are in the liquid state |
A suspension is a turbid dispersion that has large particles. |
|
Heterogeneous nature |
Homogeneous nature |
Homogeneous nature |
|
The particles are invisible to the naked eye; can observe them under an ultra-microscope |
Invisible to naked eye |
Visible to naked eye |
|
Dimensions are around 1 nanometer to 1 micrometre |
Below 1 nanometer |
Above 1 micrometre |
3. To make a saturated solution, 36 g of sodium chloride is dissolved in 100 g of water at 293 K. Find its concentration at this temperature.
Answer:-.

Textbook Page No. 24
1. How will you separate a mixture containing kerosene and petrol (difference in their Is Matter around us Pure boiling points is more than 25°C), which are miscible with each other?
Answer:-. Since the difference in the boiling points of kerosene and petrol is more than 25°C, they can be separated from the mixture by simple distillation method.
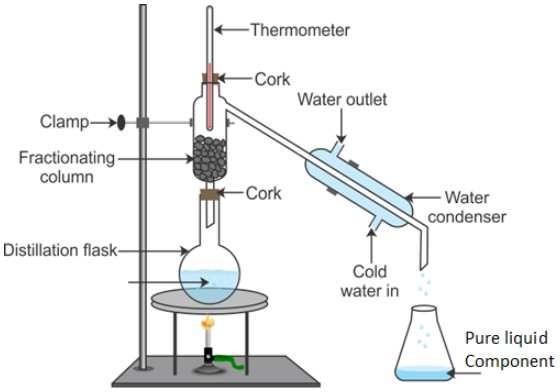
The mixture of kerosene and petrol is taken in the distillation flask. A thermometer is fitted to it. The apparatus is arranged as shown in the figure. The mixture is heated slowly by a burner keeping a close watch at the thermometer. At a certain point, the reading of the thermometer becomes constant. Petrol having the lower boiling point vaporises first and the vapours of petrol formed are passed through a condenser. In the condenser, the petrol vapours get cooled and liquefied. It can be collected from the condenser outlet in a receiver. The heating is stopped when the temperature further starts rising. Kerosene is left behind in the distillation flask.
2. Name the technique to separate
(i) Butter from curd,
Answer:-. Centrifugation.
(ii) Salt from sea-water.
Answer:-. Evaporation.
(iii) Camphor from salt.
Answer:-. Sublimation.
3. What type of mixtures are separated by the technique of crystallisation?
Answer:-. When a mixture of a solid and a liquid substance is separated using the process of evaporation, it gives only the residue which may contain some impurities. To obtain the solid free from impurities, the process of crystallisation is used. In this process, the pure solid in the form of crystals separates from a solution.
Page No. 24
1. Classify the following as chemical or physical changes:
(i) Cutting of tree.
Answer:-. Deforestation is cutting down of trees in large numbers and clearing out the forest areas. To get the resources from forests, people cut down trees, which leads to the extinction of many plant species.
(ii) Melting of butter in a plan.
Answer:-. Melting butter in a pan is known as an example of a physical change.
(iii) Rusting of almirah.
Answer:-. Rusting of the almirah is a chemical change.
(iv) Boiling of water to form steam.
Answer:-. Boiling of water to form steam is a physical change because during this change only change of state occurs from liquid water to steam (gaseous) without any change in its chemical composition.
(v) passing of electric current, through water and the water breaking down into hydrogen and oxygen gases.
Answer:-. Passing of electrical energy through water to form hydrogen and oxygen is a chemical change.
(vi) Dissolving common salt in water.
Answer:-. Dissolving common salt in water is known as an example of a physical change.
(vii) Making a fruit salad with raw fruits, andburning of paper and wood.
Answer:-. Do your selfs.
2. Try segregating the things around you as pure substances or mixtures.
Answer:-. Pure substances: Water, sugar, sodium chloride, iron, silver, gold, camphor, etc. Mixtures: Soil, minerals, soft drink, paper, salt solution, sugar solution, milk, petroleum, tea, coffee, clothes, vegetables, etc.
EXERCISES
1. Which separation techniques will you apply for the separation of the following?
(a) Sodium chloride from its solution in water.
Answer:-. Evaporation.
(b) Ammonium chloride from a mixture containing sodium chloride and ammonium chloride.
Answer:-. Sublimation.
(c) Small pieces of metal in the engine oil of a car.
Answer:-. Filtration.
(d) Different pigments from an extract of flower petals.
Answer:-. Chromatography.
(e) Butter from curd.
Answer:-: Centrifugation.
(f) Oil from water.
Answer:-. Distillation.
(g) Tea leaves from tea.
Answer:-. Filtration.
(h) Iron pins from sand.
Answer:-. Magnetic separation.
(i) Wheat grains from husk.
Answer:-. Winnowing.
(j) Fine mud particles suspended in water.
Answer:-. Centrifugation.
2. Write the steps you would use for making tea. Use the words solution, solvent, solute, dissolve, soluble, insoluble, filtrate and residue.
Answer:-. Some water (solvent) is taken in a kettle. It is heated on a burner till it boils. Some Tea leaves are added to boiling water. The tea leaves are insoluble in water. However, the colour and the flavour of tea-leaves go into the solvent. It is filtered and the filtrate is collected in a cup. The used tea-leaves are left behind in the sieve as residue. Milk (solute) is added to the filtrate and they form a solution. Now, required quantity of sugar (solute) is added to the solution. Sugar is soluble in water. Hence, the solution is stirred till the sugar gets dissolved. The solution, i.e. tea is ready to drink.
3. Pragya tested the solubility of three different substances at different temperatures and collected the data as given below (results are given in the following table, as grams of substance dissolved in 100 grams of water to form a saturated solution).
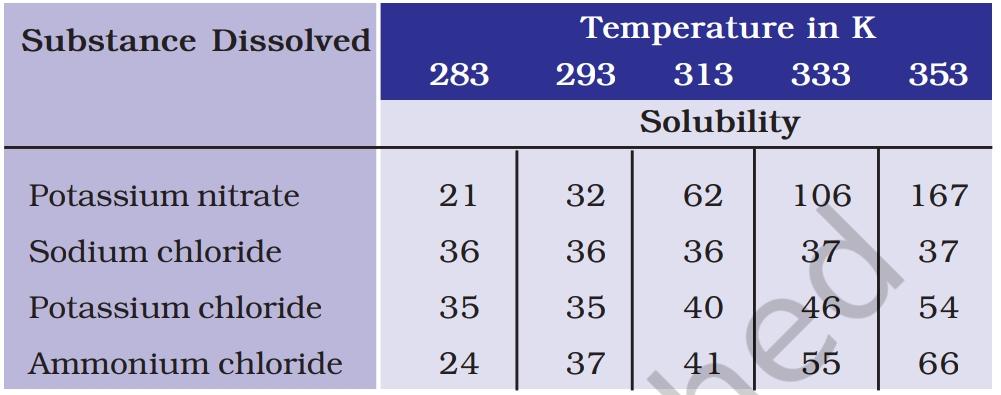
(a) What mass of potassium nitrate would be needed to produce a saturated solution of potassium nitrate in 50 grams of water at 313 K?
Answer:-. (a) From the given data, The mass of potassium nitrate needed to produce a saturated solution of potassium nitrate in 100 g of water at 313 K is 62 g.
Therefore, the mass of potassium nitrate needed to produce a saturated solution of potassium nitrate in 50g water at 313 K = 62/100×50 = 31g.
(b) Pragya makes a saturated solution of potassium chloride in water at 353 K and leaves the solution to cool at room temperature. What would she observe as the solution cools? Explain.
Answer:-: As the solution cools, Pragya would observe that crystals of potassium chloride start to form and settle at the bottom of the container. This happens because the solubility of solids in liquids generally decreases with a decrease in temperature.
At 353 K, 100 grams of water can dissolve a maximum of 54 grams of potassium chloride, whereas at room temperature (approximately 293 K), only 35 grams can dissolve. Therefore, as the solution cools from 353 K to 293 K, the excess 19 grams of potassium chloride (54 grams – 35 grams) will separate out in the form of solid crystals.
(c) Find the solubility of each salt at 293 K. Which salt has the highest solubility this temperature?
Answer:-: At 293 K:
Solubility of potassium nitrate = 32g Solubility of sodium chloride = 36g Solubility of potassium chloride = 35g Solubility of ammonium chloride = 37g
Thus, ammonium chloride has the highest solubility (37g) at 293 K.
(d) What is the effect of change of temperature on the solubility of a salt?
Answer:-: From the given data, it is seen that the solubility of a salt increases on increasing the temperature in most of the cases.
4. Explain the following giving examples.
(a) Saturated solution.
Answer:-. Saturated solution: At a particular temperature, there is a maximum limit to the amount of solute that can be dissolved in a given amount of solvent. A solution in which the maximum amount of solute is dissolved at a particular temperature is known as a saturated solution. Thus in a saturated solution, no more solute can be dissolved at the given temperature.
For example, soft drink and nitrogen in earth’s soil.
(b) Pure substance.
Answer:-: Pure substance: A substance consisting of a single type of particles is called a pure substance. The chemical nature of all the constituting particles of a pure substance is the same. A pure substance cannot be separated into other kinds of matter by any physical process. A pure substance has a fixed melting and boiling point. All the elements and compounds are pure substances.
For example, silver, gold, water, sodium chloride, etc.
(c) Colloid.
Answer:-: A colloid or colloidal solution is a heterogeneous mixture in which the particles in dispersed phase are uniformly spread in a continuous dispersion medium. For example, milk is a colloidal solution in which butter fat is dispersed in water. The properties of a colloid are intermediate between those of a solution and a suspension.
For example, blood, milk, soap solution, etc.
The characteristics of a colloid or colloidal solution are:
1. The diameter of the particles of a colloid lies between 10m and 10 m. Therefore, they cannot be seen by naked eyes. However, they are visible under ultramicroscope.
2. A colloid is trAnswer:-lucent or opaque.
3. The solute particles of a colloid can be separated from the colloid by using a special technique known as centrifugation.
4. A colloidal solution scatters a beam of light passing through it and makes visible,
i.e. it shows the Tyndall effect.
(d) Suspension.
Answer:-: Suspension: A suspension is a heterogeneous mixture in which the solute particles are dispersed throughout the medium without dissolving in it. So in suspension, the solute particles do not dissolve but remain suspended throughout the bulk of the medium. For example, muddy water, chalk-water mixture, etc.
The characteristics of suspensions are:
1. The diameter of the particles of a suspension is of the order of 10 m. Therefore, they can be seen with naked eyes.
2. A suspension is trAnswer:-lucent.
3. Solute particles of a suspension can be separate from the suspension by the process of filtration.
5. Classify each of the following as a homogenous or heterogenous mixture. soda water, wood, air, soil, vinegar, filtered tea.
Answer:-. Soda water: Homogenous mixture.
Wood: Heterogenous mixture.
Air: Homogenous mixture. Soil: Heterogenous mixture. Vinegar: Homogenous mixture.
Filtered tea: Homogenous mixture.
6. How would you confirm that a colourless liquid given to you is pure water?
Answer:-. Pure water has a boiling point of 100 degree celsius/373K. Hence, to verify if the colourless liquid is pure water, we’ll boil it and observe its boiling point. If the liquid starts boiling at a sharp 100 degree celsius/373K, then the colourless liquid will be pure water.
7. Which of the following materials fall in the category of a “pure substance”?
(a) Ice.
(b) Milk.
(c) Iron.
(d) Hydrochloric acid.
(e) Calcium oxide.
(f) Mercury.
(g) Brick.
(h) Wood.
(i) Air.
Answer:-. All the elements and compounds are pure substances. So the elements, iron and mercury and the compounds, ice, hydrochloric acid and calcium oxide are pure substances.
8. Identify the solutions among the following mixtures.
(a) Soil.
(b) Sea water.
(c) Air.
(d) Coal.
(e) Soda water.
Answer:-. Sea water, air and soda water are solutions.
9. Which of the following will show “Tyndall effects”?
(a) Salt solution.
(b) Milk.
(c) Copper sulphate solution.
(d) Starch solution.
Answer:-. Milk and starch are colloid solutions. This, they will show ”Tyndall effect”.
10. Classify the following into elements, compounds and mixtures.
(a) Sodium.
(b) Soil.
(c) Sugar.
(d) silver.
(e) Calcium carbonate.
(f) Tin.
(g) Silicon.
(h) Coal.
(i) Air.
(j) Soap.
(k) Methane.
(l) Carbon dioxide.
(m) Blood.
Answer:-.
|
Elements |
Compounds |
Mixtures |
|---|---|---|
|
(a) Sodium |
(e) Calcium carbonate |
(b) Soil |
|
(b) Silver |
(k) methane |
(c) Sugar solution |
|
(c) Tin |
(i) Carbon dioxide |
(h) Coal |
|
(d) Silicon |
(i) Air |
|
|
(j) Soap |
||
|
(m) Blood |
11. Which of the following are chemical changes?
(a) Growth of a plant.
(b) Rusting of iron.
(c) Mixing of iron filings and sand.
(d) Cooking of food.
(e) Digestion of food.
(f) Freezing of water.
(g) Burning of a candle.
Answer:-. A chemical change is usually an irreversible reaction involving the rearrangement of the atoms of one or more substances. The change occurs in the chemical properties or composition, resulting in the formation of at least one new substance. Growth of a plant, rusting of iron, cooking of food, digestion of food, and burning of a candle are chemical changes because the chemical composition of the substance changes.
SEBA Assam Class 9 Science Chapter 2 – Is Matter Around Us Pure FAQs
Get Free NCERT PDFs
If you want to download free PDFs of any chapter, click the link below and join our WhatsApp group:

