SEBA Class 9 Science Chapter 3 – Atoms and Molecules Solutions & Summary
Looking for SEBA Class 9 Science Chapter 3 – “Atoms and Molecules” solutions? At Ospin Academy, you will find NCERT-based textbook answers, multiple-choice questions (MCQs), and a detailed chapter summary to help you excel in exams.
📖 Chapter Overview:
This chapter explains the fundamental concepts of atoms, molecules, laws of chemical combination, and how they form compounds.
📌 Key Topics Covered:
- ⭐ Laws of Chemical Combination
- ⭐ Dalton’s Atomic Theory
- ⭐ What are Atoms and Molecules?
- ⭐ Atomic Mass and Molecular Mass
- ⭐ Mole Concept
📌 Important Concepts:
- ⭐ What are the postulates of Dalton’s Atomic Theory?
- ⭐ How are atoms different from molecules?
- ⭐ What is the Law of Conservation of Mass?
- ⭐ How to calculate molecular mass?
- ⭐ What is the significance of the mole concept?
📝 How Ospin Academy Helps:
- ✅ Exam-Oriented Solutions: Fully NCERT-based Class 9 Science solutions.
- ✅ MCQs and Extra Questions: Important multiple-choice questions for better revision.
- ✅ Concept Clarity: Explanation with real-life examples.
- ✅ Quick Revision Notes: Key points summarized for last-minute preparation.
Access complete SEBA Class 9 Science Chapter 3 – “Atoms and Molecules” solutions at Ospin Academy and improve your understanding today!
Class 9 Science (English Medium) PDF Solutions 2025-26 | SEBA Assam
Download Class 9 Science (English Medium) PDF with detailed solutions, MCQs, and additional practice questions for SEBA Assam 2025-26.
Class 9 Science
Chapter – 3 (Ospin Academy)
Atoms and Molecules
Textbook Page No. 32-33
1. In a reaction, 5.3 g of sodium carbonate reacted with 6 g of ethanoic acid. The products were 2.2 g of carbon dioxide, 0.9 g water and 8.2 g of sodium ethanoate. Show that these observations are in agreement with the law of conservation of mass. Sodium carbonate + ethanoic acid log → sodium ethanoate + carbon dioxide + water
Ans. Total massa of the reactants
= Mass of sodium carbonate+Mass of ethanoic acid
= 5.3g+6g
= 11.3g
Total mass of the products
= Mass of carbon dioxide + Mass of water + Mass of sodium ethanoate
= 2.2g + 0.9g + 8.2g
= 11.2g
Since the total mass of the reactants is equal to the total mass of the products, these observations are in agreement with the law of conservation of mass.
2. Hydrogen and oxygen combine in the ratio of 1:8 by mass to form water. What mass of oxygen gas would be required to react completely with 3g of hydrogen gas?
Ans. 1 g of hydrogen reacts with 8 gm of oxygen to form water. Therefore, according to the law of constant proportions, Mass of oxygen gas required to react completely with 3 g of hydrogen gas is 8 × 3g = 24 g
3. Which postulate of Dalton’s atomic theory is the result of the law of conservation of mass?
Ans. The postulate of Dalton’s atomic theory is the results of the law of conversation of mass are:
(i) All matter is composed of the indivisible particles called atoms. Atoms of an element cannot be created neither can be destroyed.
(ii) Atoms of the same element have similar shape and mass, but the mass and shape can vary for atoms of other elements.
4. Which postulate of Dalton’s atomic theory can explain the law of definite proportions?
Ans. The postulate of Dalton’s atomic theory that can explain the law of definite proportions is- “The relative mumber and kinds of atoms are constant in a given compound.”
Textbook Page No. 35
1. Define the atomic unit.
Ans. The atomic mass unit (amu) is the quantity of mass equal to exactly one-twelfth (photo) the mass of one atom of C12
One atomic mass unit (1u)
1 u = (1/12) × mass of one C12 atom
Numerically,
1 u = 1.66 × 10⁻²⁷ kg
2. Why is it not possible to see an atom with naked eyes?
Ans. An atom is considered to be the smallest unit of a matter. Its size is smaller than anything that we can imagine or compare with. If more than millions of atoms are stacked, they will make a layer barely as thick as a sheet of paper. Hence, it is not possible to see an atom with naked eyes.
Textbook Page No. 39
1. Write down formula of-
(i) Sodium oxide.
Ans. (i) Na2O.
(ii) Aluminium chloride.
Ans: AICI3.
(iii) Sodium sulphide.
Ans: Na2S.
(iv) Magnesium hydroxide.
Ans: Mg(OH)2.
2. Write down the names of compounds represented by the following formulae:
(i) AI2(SO⁴)3.
Ans. (i) Aluminium sulphate.
(ii) CaCI2.
Ans. Calcium chloride.
(iii) K2SO4.
Ans. Potassium sulphate.
(iv) KNO3.
Ans. Potassium nitrate.
(v) CaCO3.
Ans. Calcium carbonate.
3. What is meant by the term chemical formula?
Ans. The chemical formula of a compound is a symbolic representation of its composition. It indicates the number and types of atoms of different elements present in one molecule of the compound. For example, carbon dioxide is a compound made up of 1 atom of carbon and 2 atoms of oxygen.
Therefore, the formula of carbon dioxide is written as CO₂.
4. How many atoms are present in a
-
- H2S molecule.
Ans. H2S molecule: 3 atoms; 2 atoms of hydrogen and 1 atom of sulphur.
PO4³⁻ ion?
Ans: PO4³{O ion: 5 atoms; 1 atom of phosphorus and 4 atoms of oxygen.
Textbook Page No. 40
1. Calculate the molecular masses of H2, O2, CI2, CO2, CH4, C2H₆, C2H4, NH3, CH3OH.
Ans. Molecular mass of H2 = 2 × atomic of H
= 2 × 1
= 2u
Molecular mass of O2 = 2 × atomic mass of O
= 2 × 16
= 32u
Molecular mass of CI² = 2 × atomic mass of CI
= 2×35.5
= 71u
Molecular mass of CO2 = (1×atomic mass of C) + (2 × atomic mass of oxygen)
= (1 × 12)̇ + (2 × 16)
= 12 + 32
= 44u
Molecular mass of CH4 = (1 x atomic mass of C) + (4 x atomic mass of hydrogen)
= (1 × 12) + (4 × 1)
= 12 + 4
= 16u
Molecular mass of C2H₆ = (2x atomic mass of C) + (6 × atomic mass of H)
= (2 × 12)+(6 × 1)
= 24 + 6
= 30u
Molecular mass of C2H4 = (2 × atomic mass of C) + (4 x atomic mass of H)
= (2 × 12) + (4 × 1)
= 24 + 4
= 28u
Molecular mass of NH3 = (1 × atomic mass of N) + (3 x atomic mass of H)
= (1 × 14)+(3 × 1)
= 14 + 3
= 17 u
Molecular mass of CH3OH
= (1x atomic mass of C) + (3 x atomic mass of H)+(1x atomic mass of O) + (1x atomic mass of H)
= (1 × 12)+(31)+(1 × 16) + (1 × 1) =
=12 + 3 + 16 + 1
= 32u.
2. Calculate the formula unit masses of ZnO, Na2O, K2CO3, given atomic masses of Zn = 65u, Na = 23 u, K=39 u, C= 12 u and O = 16 u.
Ans. Formula unit mass of ZnO = (1 × atomic mass of Zn) + (1 x atomic mass of O)
= (1 × 65) + (1 × 16)
= 65+16
= 81 u
Formula unit mass of Na2O = (2 × atomic mass of Na) + (1 × atomic mass of O) (2 × 23) + (1 × 16)
= 46 + 16
= 62 u
Formula unit mass of K2CO3 = (2 × atomic mass of K) + (1 × atomic mass of C) + (3
× atomic mass of O)
= (2 × 39) + (1 × 12) + (3 × 16)
= 78 + 12 + 48
= 138u.
Textbook Page No. 42.
1. If one mole of carbon atoms weighs 12 grams, what is the mass (in grams) of 1 atom of carbon?
Ans.
The mass of one mole of carbon atoms = 12 g
The number of atoms in one mole = 6.022 x 1023 atoms
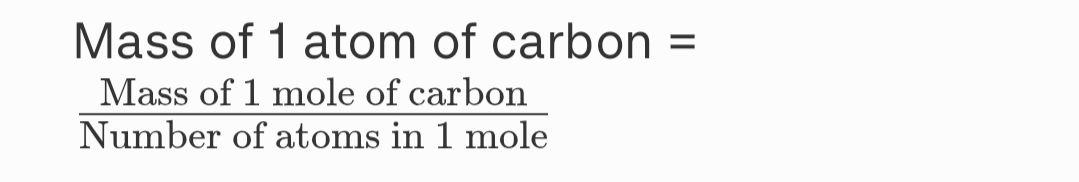
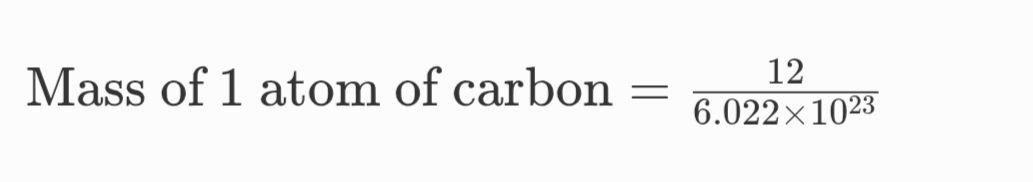
Mass of 1 atom of carbon = 1.993 × 1023 g
Thus, the mass of 1 atom of carbon is 1.993 x 1023 grams.
2. Which has more number of atoms, 100 grams of sodium or 100 grams of iron (given, atomic mass of Na= 23 u, Fe = 56 u)
Ans.
Atomic mass of Sodium (Na) = 23 u
Atomic mass of Iron (Fe) = 56 up
Avogadro’s number = 6.022 x 10^23
Calculations:
For Sodium (Na):
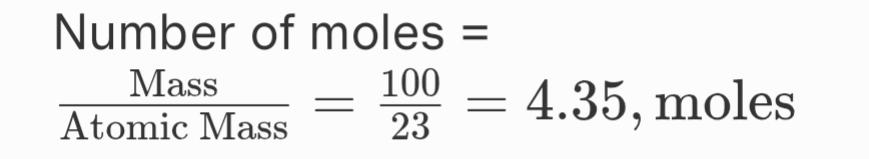
Total number of atoms = 4.35, moles x 6.022 x 1023 = 2.62 x 1024, atoms
For Iron (Fe):
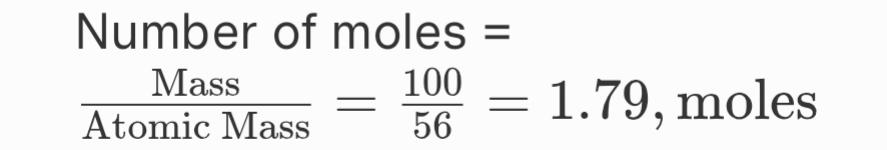
Total number of atoms = 1.79, moles x 6.022 x 1023 = 1.08 x 1024, atoms
hence,
100 grams of sodium has more atoms (2.62 x 1024) compared to 100 grams of iron (1.08 x 1024).
(Textbook Page No. 43-44)
1. A 0.24 g sample of compound of oxygen and boron was found by analysis to contain 0.096 g of boron and 0.144 g of oxygen. Calculate the percentage composition of the compound by weight.
Ans. Given:
Total mass of the compound = 0.24 g
Mass of Boron (B) = 0.096 g
Mass of Oxygen (O) = 0.144 g
Formula:
Percentage composition of an element = 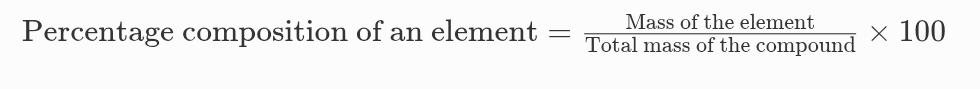
Now,
Percentage of Boron (B):
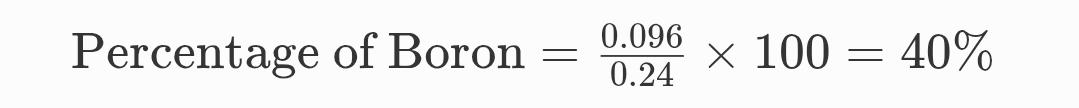
And,
Percentage of Oxygen (O):
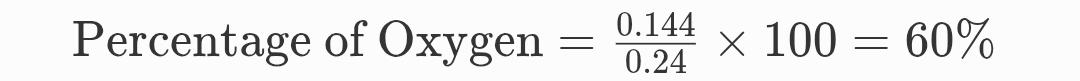
So,
Percentage of Boron = 40%
Percentage of Oxygen = 60%
2. When 3.0 g of carbon is burnt in 8.0 g of oxygen, 11.0 g of carbon dioxide is produced. What mass of carbon dioxide will be formed when 3.0 g of carbon is burnt in 50.0 g of oxygen? Which law of chemical combination will govern your answer?
Ans.
From the given data:
Mass of carbon dioxide = Mass of carbon + Mass of oxygen reacted = 3.0 g + 8.0 g = 11.0 g
If 3.0 g of carbon is burnt in 50.0 g of oxygen, only 8.0 g of oxygen will react, and the remaining oxygen will be unreacted.
The mass of carbon dioxide formed will still be 11.0 g.
Law of Chemical Combination:
The above reaction follows the Law of Conservation of Mass, which states that mass can neither be created nor destroyed in a chemical reaction.
3. What are polyatomic ions? Give examples.
Ans.
Polyatomic ions are ions that consist of two or more atoms chemically bonded together, carrying a net positive or negative charge.
Examples:
SO₄²⁻ (Sulfate ion)
NO₃⁻ (Nitrate ion)
NH₄⁺ (Ammonium ion)
4. Write the chemical formulae of the following:
Ans.
Magnesium chloride: MgCl₂
Calcium oxide: CaO
Copper nitrate: Cu(NO₃)₂
Aluminium chloride: AlCl₃
Calcium carbonate: CaCO₃
5. Give the names of the elements present in the following compounds:
Ans.
a) Quick lime (CaO): Calcium (Ca) and Oxygen (O)
b) Hydrogen bromide (HBr): Hydrogen (H) and Bromine (Br)
c) Baking powder (NaHCO₃): Sodium (Na), Hydrogen (H), Carbon (C), and Oxygen (O)
d) Potassium sulphate (K₂SO₄): Potassium (K), Sulphur (S), and Oxygen (O)
6. Calculate the molar mass of the following substances:
Ans.
a) Ethyne (C₂H₂):
Molar mass = (2 × 12) + (2 × 1) = 24 + 2 = 26 g/mol
b) Sulphur molecule (S₈):
Molar mass = 8 × 32 = 256 g/mol
c) Phosphorus molecule (P₄, atomic mass of phosphorus = 31):
Molar mass = 4 × 31 = 124 g/mol
d) Hydrochloric acid (HCl):
Molar mass = (1 × 1) + (1 × 35.5) = 36.5 g/mol
e) Nitric acid (HNO₃):
Molar mass = (1 × 1) + (1 × 14) + (3 × 16) = 63 g/mol
SEBA Assam Class 9 Science Chapter 3 – Atoms and Molecules FAQs
Get Free NCERT PDFs
If you want to download free PDFs of any chapter, click the link below and join our WhatsApp group:

